Water quality is an important concern when it comes to humidification. Regardless of what type of humidification you’re using (isothermal or adiabatic), chances are you will need to add some degree of water treatment.
Let’s consider water treatment first in terms of the humidification method being used.
Untreated Water in Isothermal and Adiabatic Applications
If isothermal humidification is used and we’re changing water into steam, all of the minerals and chemicals in the water are going to precipitate out of the liquid and adhere to the heat exchange surfaces. Obviously, this isn’t good for the life of the equipment, nor is it good for the heat transfer capability. Untreated water can add a costly burden in terms of inefficiency, as well as maintenance and premature replacement of parts and equipment.
Untreated water in adiabatic applications adds another layer of concern because we’re spraying water directly into the supply air, which means that building occupants could be inhaling the minerals and chemicals in the water. In an industrial application, the particulate ends up in the process.
Water Quality for Humidification
The industry recognizes these four basic levels of water quality when it comes to humidification:
Potable Water
Potable water is water straight from the tap or well, i.e. water we drink. This water may contain any number of living microorganisms, dissolved organic material and minerals, as well as suspended materials. If isothermal humidification is used, all microorganisms will be killed by the boiling process. This, however, would not be the case in adiabatic humidification, which does not involve adding heat to create steam.
In most commercial applications, potable water isn’t suitable for any type of sustained humidification. The water hardness alone (the presence of magnesium, calcium, iron, and silicon) will likely cause significant scale buildup, inefficiency, and downtime.
Softened Water
Water softening is a technique to improve water quality by which common “hard” elements (primarily calcium and magnesium) are removed from the water via a process that involves exchanging these elements for something else, usually sodium. Without going into too much detail, water softening relies on a tried and true “ion exchange” process in which calcium and magnesium dissolve into positively charged ions, creating an ion exchange environment which causes these elements to exit the water. There are various brands of water softening equipment on the market, but all rely on the same principle.
Water softening is typically very effective, and can be expected to extend maintenance intervals from yearly to every three years. Water softening should be applied any time water hardness exceeds 12 grains per gallon.
Water softening is considered a minimum strategy for commercial humidification.
Reverse Osmosis
For applications requiring a higher level of water purity, a process called “reverse osmosis” (RO) can be used as a second step after water softening. Reverse osmosis involves driving the already softened water through a semipermeable membrane using pressure. There are low capacity standalone RO systems that will effectively remove 97% or more of total dissolved solids from the water.
This process would be considered a minimum purification level for any humidification applications involving the manufacture of semiconductors, electronic equipment, or pharmaceuticals, as well as laboratory and health care applications.
Deionized (DI) Water
For the most critical applications, chemical deionization, can be used as an additional final step to “polish” water before sending it to any humidification equipment. Chemical deionization involves the use of ion-exchange resins to exchange hydrogen and hydroxide ions for dissolved minerals. This method is extremely effective at removing virtually all dissolved minerals from the water. DI water is pure, meaning it will not conduct electricity, and is rated as such in terms of its resistance in Meg-Ohms. The higher the resistance value in Meg-Ohms, the purer the water. Deionized water will have a resistivity rating up to 18 Meg-Ohms.
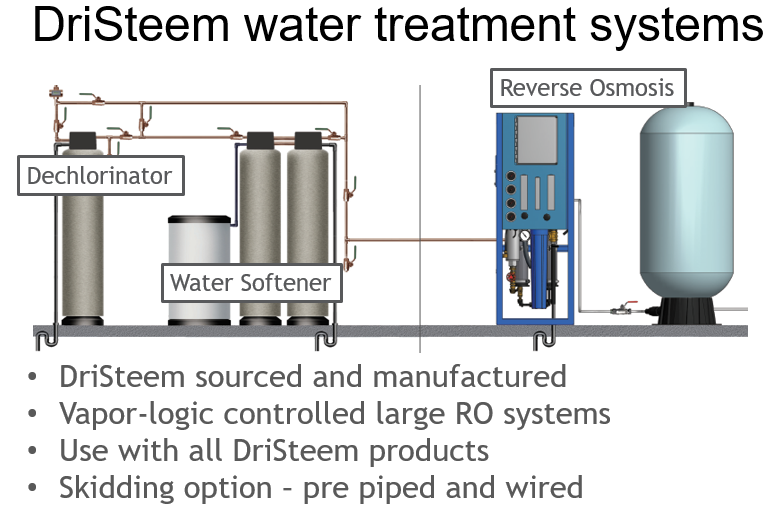
For design simplicity, DriSteem offers water treatment systems that incorporate any or all the above solutions into prepiped, preskidded units. These types of systems bring most of the humidification maintenance to a single point versus all the humidification connected equipment. These systems can be as simple or complex as the application demands.
Catch up on the entire series:
Part One: Humidification Basics: Why We Humidify in Building Design
Part Two: Humidification Basics Part 2: Mastering Humidification Terms
Part Three: Psychrometrics Made Easy: Humidification Basics Part 3
Part Four: Calculating Natural and Mechanical Loads: Humidification Basics Part 4
Part Five: Calculating Humidification Loads for Economizer Cycle Systems: Humidification Basics Part 5
Part Six: Steam Isothermal Humidification: Humidification Basics Part 6
Part Seven: Load Calculation using Dristeem DriCalc: Humidification Basics Part 7
Part Eight: Adiabatic Humidification: Humidification Basics Part 8